Abstract
Introduction: N8-GP is an extended half-life recombinant factor VIII product indicated for use in patients with hemophilia A. The pathfinder trials evaluated routine prophylaxis and bleed control in previously treated patients aged ≥12 years (pathfinder2) and patients aged <12 years (pathfinder5), including an extension trial (pathfinder8). The objective of the present analysis was to compare the efficacy and safety of N8-GP in US vs global (including US) patients.
Methods: In the pathfinder2 trial, patients aged ≥12 years with severe hemophilia A were administered N8-GP 50 IU/kg every 4 days (Q4D) as routine prophylaxis or 20 to 70 IU/kg as on-demand treatment. Patients aged <12 years received prophylaxis with N8-GP 60 IU/kg twice weekly in the pathfinder5 trial, and bleeding episodes were treated with 20 to 75 IU/kg. In the pathfinder8 extension trial, the investigator decided on which treatment arm the patient should be allocated based on previous treatment regimen and taking into account bleeding tendency.
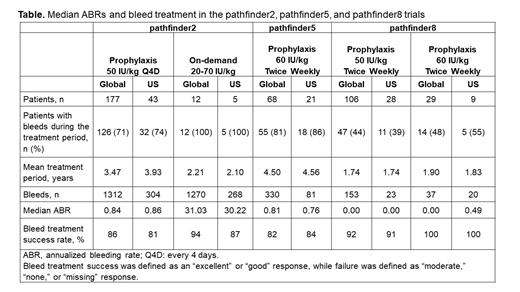
Results: See Table for efficacy in prophylaxis (median annualized bleeding rates [ABRs]) and bleed treatment (success rate). Median ABRs tended to decrease from 0.84 (global) and 0.86 (US) in pathfinder2 to 0.00 (global) and 0.00 (US) in pathfinder8 in patients aged ≥12 years on the prophylaxis regimen. In addition, in patients aged <12 years, median ABRs decreased from 0.81 (global) and 0.76 (US) in pathfinder5 to 0.00 (global) and 0.49 (US) in pathfinder8. Treatment success rates for US patients were comparable to those for global patients across trials and 100% were rated "excellent" or "good" for patients aged <12 years in pathfinder8.
Overall, there were 59 treatment-related adverse events (AEs) in patients on the prophylactic regimen in pathfinder2, 13 of which occurred in US patients, and 11 treatment-related AEs in patients treated on-demand, 3 of which occurred in US patients. In pathfinder5, there were 16 treatment-related AEs, 7 of which occurred in US patients. In pathfinder8, there were 6 treatment-related AEs in patients aged ≥12 years, 3 of which were in US patients. In addition, there was 1 treatment-related AE in a non-US patient aged <12 years. AEs included rash, redness, and injection site reactions. Across trials, 1 previously treated patient (an 18-year-old, from a US site) with an intron 22 inversion developed a low-titer inhibitor at 93 exposure days to N8-GP and was withdrawn when the titer progressed to >5 Bethesda units. The patient returned to their previous FVIII product, and their inhibitor status was negative at follow-up.
Conclusions: Efficacy of N8-GP was sustained during the main and extension pathfinder trials in both global and US patients. In addition, a favorable safety profile was maintained throughout the course of the program.
Wheeler: Novo Nordisk A/S: Consultancy; Bayer: Consultancy; BioMarin: Consultancy; HEMA Biologics: Consultancy; Spark: Consultancy; Takeda: Consultancy; UniQure: Consultancy. Kearney: Grifols: Research Funding; Daiichi Sankyo: Research Funding; CVS Pharmacy: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novo Nordisk Inc: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Bioverativ/Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Staber: Bayer, CSL Behring, Sanofi, Takeda: Membership on an entity's Board of Directors or advisory committees. Wufsus: Novo Nordisk Inc: Current Employment. Ostrow: Novo Nordisk Inc: Current Employment. Lentz: Novo Nordisk Inc: Consultancy, Honoraria, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal